Serial and Parallel Battery Configurations
Battery packs achieve the desired operating voltage by connecting several cells in series, with each cell adding to the total terminal voltage. Parallel connection gives higher capacity for increased current handling, as each cell adds to the total current handling. Some packs may have a combination of serial and parallel connections. Laptop batteries commonly have four 3.6V Li-ion cells in series to achieve 14.4V and two strings of these 4 cells in parallel (for a pack total of 8 cells) to boost the capacity from 2,400mAh to 4,800mAh. Such a configuration is called 4S2P, meaning 4 cells are in series and 2 strings of these in parallel. Insulating foil between the cells prevents the conductive metallic skin from causing an electrical short. The foil also shields against heat transfer should one cell get hot.
Most battery chemistries allow serial and parallel configuration. It is important to use the same battery type with equal capacity throughout and never mix different makes and sizes. A weaker cell causes an imbalance. This is especially critical in a serial configuration and a battery is only as strong as the weakest link.
Imagine a chain with strong and weak links. This chain can pull a small weight but when the tension rises, the weakest link will break. The same happens when connecting cells with different capacities in a battery. The weak cells may not quit immediately but get exhausted more quickly than the strong ones when in continued use. On charge, the low cells fill up before the strong ones and get hot; on discharge the weak are empty before the strong ones and they are getting stressed.
Single Cell Applications
The single-cell design is the simplest battery pack. A typical example of this configuration is the cellular phone battery with a 3.6V lithium-ion cell. Other uses of a single cell are wall clocks, which typically use a 1.5V alkaline cell, as well as wristwatches and memory backup.
The nominal cell voltage of nickel is 1.2V. There is no difference between the 1.2V and 1.25V cell; the marking is simply preference. Whereas consumer batteries use 1.2V/cell as the nominal rating, industrial, aviation and military batteries adhere to the original 1.25V. The alkaline delivers 1.5V, silver-oxide 1.6V, lead acid 2V, primary lithium 3V, Li-phosphate 3.3V and regular lithium-ion 3.6V. Li-manganese and other lithium-based systems sometimes use 3.7V. This has nothing to do with electrochemistry and these batteries can serve as 3.6V cells. Manufacturers like to use a higher voltage because low internal resistance causes less of a voltage drop with a load.
Serial Connection
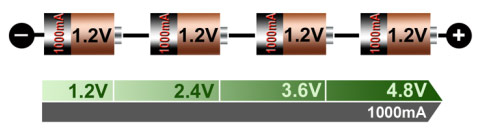
Portable equipment needing higher voltages use battery packs with two or more cells connected in series. Figure 11 shows a battery pack with four 1.2V nickel-based cells in series to produce 4.8V. In comparison, a four-cell lead acid string with 2V/cell will generate 8V, and four Li-ion with 3.6V/cell will give 14.40V. If you need an odd voltage of, say, 9.5 volts, you can connect five lead acid, eight NiMH/NiCd), or three Li-ion in series.
Figure 1: Serial connection of four NiCd or NiMH cells
Adding cells in a string increases the voltage; the current remains the same.
Courtesy of Cadex
Parallel Connection
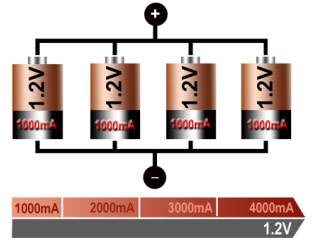
If higher currents are needed and larger cells with increased ampere-hour (Ah) ratings are not available or the design has constraints, one or more cells are connected in parallel. Most chemistries allow parallel configurations with little side effect. Figure 12 illustrates four cells connected in parallel. The voltage of the illustrated pack remains at 1.2V, but the current handling and runtime are increased fourfold.
Figure 12: Parallel connection of four cells
With parallel cells, the current handling and runtime increases while voltage stays the same.
Courtesy of Cadex
Serial/Parallel Connection
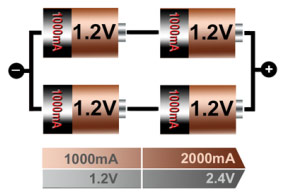
The serial/parallel configuration shown in Figure 13 allows superior design flexibility and can give the voltage and current ratings desired using standard cell sizes. The total power is the product of voltage times current, and the four 1.2V/1000mAh cells produce 4.8Wh. Serial/parallel connections are common with lithium-ion, especially for laptop batteries, and the built-in protection circuit must monitor each cell individually. Integrated circuits (ICs) designed for various cell combinations simplify the pack design.
Figure 5: Serial/ parallel connection of four cells
This configuration provides maximum design flexibility.
Courtesy of Cadex
Toby Kilroy
Writer at POTVToby has been vaping since early 2012 and has used an array of devices and kit in that time. He sometimes writes up reviews but is often found with his head stuck in pages of code with a confused smile on his face. Toby also helps run his wife's site gethistory.co.uk and has two children. He sometimes fondly remembers having free time and occasionally manages to sneak away to put his head into a good book!
Join the discussion
Electricity & Ohm's Law
A basic introduction in order to develop and appreciation for electricity and to build safe coils.
Battery safety and charging
Protect yourself and your equipment when dealing with batteries